››Convert moles Oxygen to gram
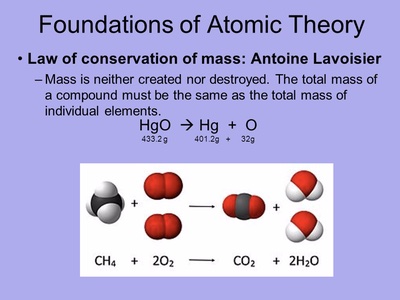
To calculate the atomic mass of oxygen using the data in the above table, we must first multiply the mass of each isotope by its corresponding natural abundance (percentage abundance). But, since the abundance is in%, you must also divide each abundance value by 100. Oxygen-15 atom is the radioactive isotope of oxygen with relative atomic mass 15.003065. The longest-lived oxygen radionuclide with half-life of 122.2 s. The atomic mass of oxygen is 15.9994 atomic mass units. Oxygen has an atomic number of 8 and is classified as a non-metal. Its symbol is O. Oxygen was discovered in 1774 by Joseph Priestly, according to Chemical Elements. Physicists, however, dealing with atoms and not reagents, required a unit that distinguished between isotopes. At least as early as 1927 6 physicists were using an atomic mass unit defined as equal to one-sixteenth of the mass of the oxygen-16 atom (the isotope of. How many moles Oxygen in 1 grams? The answer is 0.37894. We assume you are converting between moles Oxygen and gram. You can view more details on each measurement unit: molecular weight of Oxygen or grams The molecular formula for Oxygen is O. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Oxygen,.
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
››More information from the unit converter
How many moles Oxygen in 1 grams?The answer is 0.062502343837894.
We assume you are converting between moles Oxygen and gram.
You can view more details on each measurement unit:
molecular weight of Oxygen orgrams
The molecular formula for Oxygen is O.
The SI base unit for amount of substance is the mole.
1 mole is equal to 1 moles Oxygen, or 15.9994 grams.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles Oxygen and gram.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of moles Oxygen to grams
1 moles Oxygen to grams = 15.9994 grams
2 moles Oxygen to grams = 31.9988 grams
3 moles Oxygen to grams = 47.9982 grams
4 moles Oxygen to grams = 63.9976 grams
5 moles Oxygen to grams = 79.997 grams
6 moles Oxygen to grams = 95.9964 grams
7 moles Oxygen to grams = 111.9958 grams
8 moles Oxygen to grams = 127.9952 grams
9 moles Oxygen to grams = 143.9946 grams
10 moles Oxygen to grams = 159.994 grams
››Want other units?
You can do the reverse unit conversion fromgrams Oxygen to moles, or enter other units to convert below:
››Common amount of substance conversions
moles Oxygen to molecule
moles Oxygen to mole
moles Oxygen to millimol
moles Oxygen to picomol
moles Oxygen to atom
moles Oxygen to decimol
moles Oxygen to nanomol
moles Oxygen to kilomol
moles Oxygen to micromol
moles Oxygen to centimol
››Details on molecular weight calculations
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
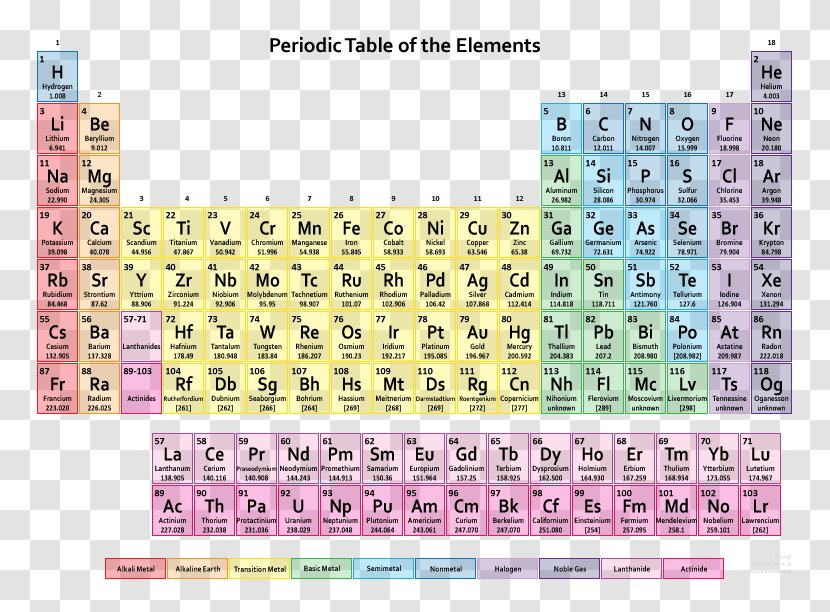
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
Dry air is a mixture of gases where the average molecular weight (or molar mass) can be calculated by adding the weight of each component
The molecular weight (or molar mass) of a substance is the mass of one mole of the substance, and can be calculated by summarizing the molar masses of all the atoms in the molecule.
Components in Dry Air
Air is a mixture of several gases, where the two most dominant components in dry air are 21 vol% oxygen and 78 vol%nitrogen. Oxygen has a molar mass of 15.9994 g/mol and nitrogen has a molar mass of 14.0067 g/mol. Since both of these elements are diatomic in air - O2 and N2, the molar mass of oxygen gas is aprox. 32 g/mol and the molar mass of nitrogen gas is aprox. 28 g/mol.
Atomic Mass Of Oxygen Atom
The average molar mass is equal to the sum of the mole fractions of each gas multiplied by the molar mass of that particular gas:
Mmixture = (x1*M1 + ......+ xn*Mn) (1)
where

xi = mole fractions of each gas
Mi = the molar mass of each gas
The molar mass of dry air with oxygen, nitrogen and the other components as indicated below is 28.9647g/mol. Composition and content of each gas in air is given in the figures and the table below.
See also AirDensity at varying pressure,Density and specific weight at varying temperature, Diffusion Coefficients for Gases in Air, Dynamic (absolute) and kinematic viscosity, Prandtl Number, Specific heat at varying temperature and Specific heat at varying pressure, Thermal Conductivity, Thermal Diffusivity, Properties at gas-liquid equilibrium conditions and Air properties, for other properties of air.
For full table with Molar mass an Molar mass in Air - rotate the screen!
| Components in dry air | Volume ratio = Molar ratio, compared to dry air | Molar Mass | Molar mass in air | |||
| Name | Formula | [mol/molair] | [vol %] | [g/mol] [kg/kmol] | [g/molair] [kg/kmolair] | [wt %] |
| Nitrogen | N2 | 0.78084 | 78.084 | 28.013 | 21.873983 | 75.52 |
| Oxygen | O2 | 0.20946 | 20.946 | 31.999 | 6.702469 | 23.14 |
| Argon | Ar | 0.00934 | 0.934 | 39.948 | 0.373114 | 1.29 |
| Carbon dioxide | CO2 | 0.00033 | 0.033 | 44.010 | 0.014677 | 0.051 |
| Neon | Ne | 0.00001818 | 0.001818 | 20.180 | 0.000367 | 0.0013 |
| Helium | He | 0.00000524 | 0.000524 | 4.003 | 0.000021 | 0.00007 |
| Methane | CH4 | 0.00000179 | 0.000179 | 16.042 | 0.000029 | 0.00010 |
| Krypton | Kr | 0.0000010 | 0.0001 | 83.798 | 0.000084 | 0.00029 |
| Hydrogen | H2 | 0.0000005 | 0.00005 | 2.016 | 0.000001 | 0.000003 |
| Xenon | Xe | 0.00000009 | 0.000009 | 131.293 | 0.000012 | 0.00004 |
| Average molar mass of air | 28.9647 | |||||
Atomic Mass Of Oxygen In Amu
- 1 kg = 2.2046 lb
Air Density
The density of dry air can be calculated with the Ideal Gas Law
ρ = P / (R T) (1)
where
P = pressure (Pa)
Rair = 287.05 = individual gas constant [J/kg K]
T = absolute temperature [K]
Example: The density of dry air at atmospheric pressure 101.325 kPa (101325 Pa) and 0 oC (= 273.15 K) can be calculated as
ρ = 101325 [Pa] / (287.05 [J/kg K]*273.15 [K])
= 1.292 [kg/m3]

Water Vapor
Water vapor is almost always present in air. The content may vary and the maximum amount possible of water vapor in dry air depends on the temperature of the air.
Water vapor - H2O - is composed of one oxygen atom and two hydrogen atoms. Hydrogen is the lightest element with a molar mass of 1 g/mol, while oxygen has 16 g/mol. Thus, the water vapor atom has an molar mass of 18 g/mol, and water vapor is lighter than O2 with 32 g/mol and N2 with 28 g/mol.
- Note! Water vapor in air will dilute the other gases and reduce the total density of the mixture. Dry air is more dense than humid air!
The vapor in air may saturate to droplets when temperature is decreased or pressure is increased.
Humid air containing water molecules as liquid - droplets - may be more dense than dry air or humid air containing water only as vapor.
Related Topics
- Air Psychrometrics - The study of moist and humid air - psychrometric charts, Mollier diagrams, air-condition temperatures and absolute and relative humidity and moisture content
- Material Properties - Material properties for gases, fluids and solids - densities, specific heats, viscosities and more
- Fluid Mechanics - The study of fluids - liquids and gases. Involves velocity, pressure, density and temperature as functions of space and time
- Gases and Compressed Air - Air, LNG, LPG and other common gas properties, pipeline capacities, sizing of relief valves
Related Documents
- Air - Composition and Molecular Weight - Dry air is a mechanical mixture of nitrogen, oxygen, argon and several other gases in minor amounts
- Air - Density at varying pressure and constant temperatures - Figures and tables showing changes in air density at pressure varying from 1 to 10 000 bara (14.5 - 145000 psi) and constant, selected temperatures. Figures are given in different scales.
- Air - Density, Specific Weight and Thermal Expansion Coefficient at Varying Temperature and Constant Pressures - Online calculator, figures and tables showing density, specific weight and thermal expansion coefficient of air at temperatures ranging -100 to 1600 °C (-140 to 2900 °F) at atmospheric and higher pressure - Imperial and SI Units
- Air - Diffusion Coefficients of Gases in Excess of Air - Diffusion coefficients, D12, for gases in large excess of air at temperature varying from 0 - 400 °C
- Air - Dynamic and Kinematic Viscosity - Online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of air at temperatures ranging from -100 to 1600°C (-150 to 2900°F) at pressure ranging from 1 to 10 000 bara (14.5 - 145000 psia) - SI and Imperial Units
- Air - Prandtl Number - Figures and table showing changes in Prandtl number for air with changes in temperature and pressure
- Air - Properties at Gas-Liquid Equilibrium Conditions - Figures and tables showing how the properties of air changes along the boiling and condensation curves (temperature and pressure between triple point and critical point conditions). An air phase diagram are also given.
- Air - Specific Heat at Constant Pressure and Varying Temperature - Online calculator, figures and tables showing how specific heat (Cp and Cv) of dry air vary with temperature at different pressures, SI and imperial units
- Air - Specific Heat at Constant Temperature and Varying Pressure - Figures and table showing isobaric (Cp) and isochoric (Cv) specific heat of air at constant temperature and varying pressure ranging 0.01 to 10000 bara
- Air - Thermal Conductivity - Online calculator, figures and tables showing air thermal conductivity at varying temperature and pressure, SI and imperial units.
- Air - Thermal Diffusivity - Figures and tables showing dry air thermal diffusivity at varying temperarure and pressure, SI and Imperial units
- Air - Thermophysical Properties - Thermal properties of air - density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusicity, and more
- Air Specific Heat Ratio - Specific Heat Ratio of air at temperatures from -40 - 1000oC (-40 - 1500oF) at standard atmospheric pressure - Imperial and SI Units
- Density of Moist Humid Air - Density of dry air and water vapor - moist or humid air
- Dry Air and Water Vapor - Density and Specific Volume - Imperial Units - Density and specific volume of dry air and water vapor at temperatures ranging 225 to 900 degF (107 to 482 degC)
- Dry Air Properties - Dry air properties at temperatures ranging 175 - 1900 K - specific heat, ratio of specific heats, dynamic viscosity, thermal conductivity, Prandtl number, density and kinematic viscosity
- Gas Mixture Properties - Special care must be taken for gas mixtures when using the ideal gas law, calculating the mass, the individual gas constant or the density
- Gases - Densities - Densities and molecular weights of some common gases - acetylene, air, methane, nitrogen, oxygen and others ..
- Gases - Molar Specific Heat - Molar specific heats of gases at constant volume
- Humid Air and the Ideal Gas Law - Pressure, temperature and volume for an ideal or perfect gas like air with water vapor - or moist air
- Ideal Gas Law - The relations between volume, pressure, temperature and quantity of a gas, including definition of density of a gas
- Mole and Avagadro's Number - The mole is the SI base unit for amount of substance
- Mole Fraction of Water Vapor in Moist Air - Mole fraction of water vapor is the ratio of water molecules - to air and water molecules
- Molecular Weight of some Common Substances - Definition and molecular weight (molar mass) of some common substances
- Nitrogen - Thermophysical Properties - Chemical, Physical and Thermal Properties of Nitrogen - N2
- Non-ideal gas - Van der Waal's Equation and Constants - Listing of van der Waals constants for more than 200 gases, used to correct for non-ideal behavior of gases caused by intermolecular forces and the volume occupied by the gas particles
- Oxygen - Thermophysical properties - Chemical, Physical and Thermal Properties of Oxygen - O2
- Speed of Sound in Air - Speed of sound in air at temperatures from -40 to 1000oC (-40 to 1500oF) at standard atmospheric pressure - Imperial and SI Units
- Total and partial pressure - Dalton's law of partial pressures - How to calculate total pressure and partial pressures for gas mixtures from Ideal Gas Law
- Universal and Individual Gas Constants - The Universal and Individual Gas Constants in fluid mechanics and thermodynamics. Individual gas constant is given for the most common gases.
Tag Search
- en: molecular mass air
- es: aire masa molecular
- de: Molekularmasse Luft
